Stress granules (SGs) are membrane-less organelles that transiently arise in the cytoplasm in response to adverse environmental conditions. They form through liquid-liquid phase separation (LLPS). SGs are thought to regulate mRNA functions during stress and participate in cell-fate specification by orchestrating the depletion and release of cytosolic regulatory proteins. Much effort has been focused on understanding the functions and regulation of SGs. SGs are highly dynamic, constantly exchanging constituents with surrounding cellular compartments. Nevertheless, the temporal landscape of SG constituents between their assembly and disassembly phases remains poorly characterized
The group led by Assistant Professor Bai Yun and Associate Professor Liu Yanfen in SLST provides a systematic depiction of the proteome profile throughout the acute to prolonged heat-shock SG life cycle. Their results were published in an article titled “Time-resolved proteomic profiling reveals compositional and functional transitions across the stress granule life cycle” in the journal Nature Communications on November 27.

In this study, the authors employed two distinct SG marker proteins, G3BP1 and CAPRIN1, to enrich time-resolved SG proteins during acute to prolonged heat-shock exposure. Following TMT-labeling of peptides, the quantitative LC-MS/MS analysis was conducted. Unsupervised hierarchical clustering analysis revealed a classification of SG proteins into early and late groups based on sample timing. A comprehensive comparison of these two protein groups revealed noteworthy differences including isoelectric point, annotated RBPs, IDR and PrLD scores, PPI network, and GO enrichment. The authors proposed a model that SG proteins orchestrate a transition from early SGs that are enriched in RNA-centric functions, to late SGs that exhibit enrichment in a diverse range of stress response-related functions. According to this model, late proteins are recruited to already-established SG platforms as discrete structural and functional modules.
The authors verified these early- and late-dynamic trends through immunoblotting and immunofluorescence analysis on various target SG proteins. Interestingly, they identified a cluster of early proteins, specifically non-muscle myosin II (NM II), known for its involvement in material transport along microfilament. Notably, both the inhibition and activation-mimic of NM II were found to influence SGs assembly, underscoring the vital role of the NM II complex in the formation, mobility, and coalescence of SG.
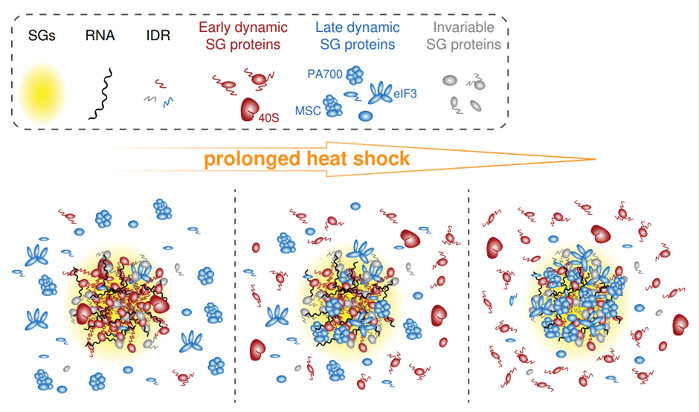
Fig.1 A model for SG compositional transitions under prolonged heat-shock.
Hu Shuyao, PhD candidate in SLST is the first author of the paper. Prof. Bai Yun and Prof. Liu Yanfen, and Hu Shuyao are the corresponding authors.
*This article is provided by Prof. Liu Yanfen

